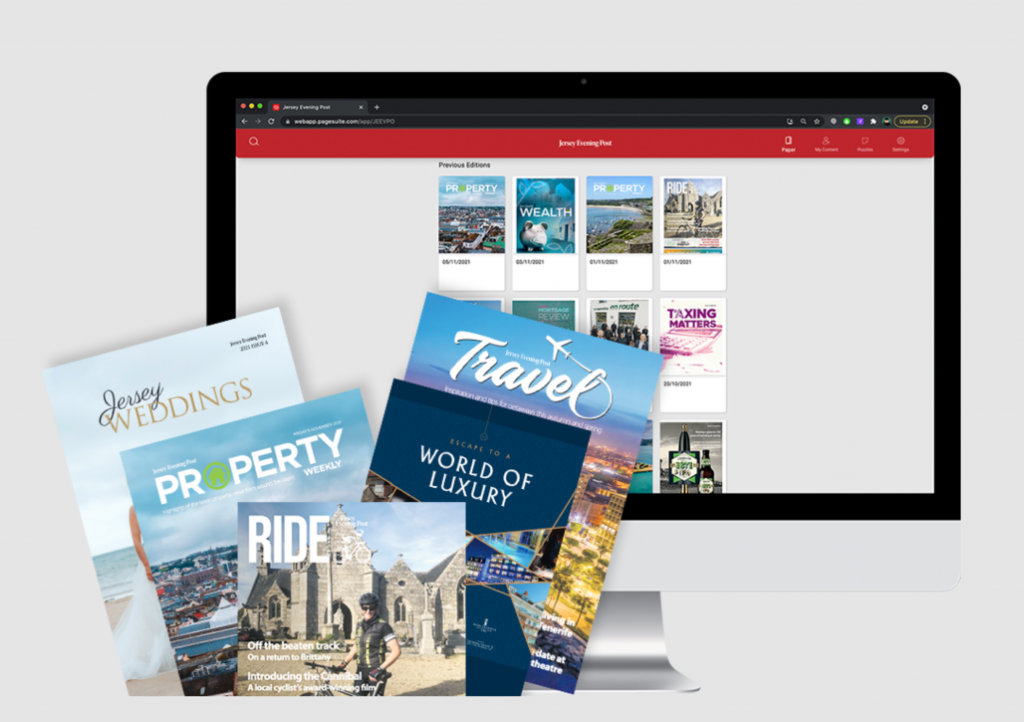
A coroner has warned that more babies could die from issues with unlicensed medicines if providers do not face stricter requirements to report problems following an inquest which concluded three infants died after receiving contaminated feed.
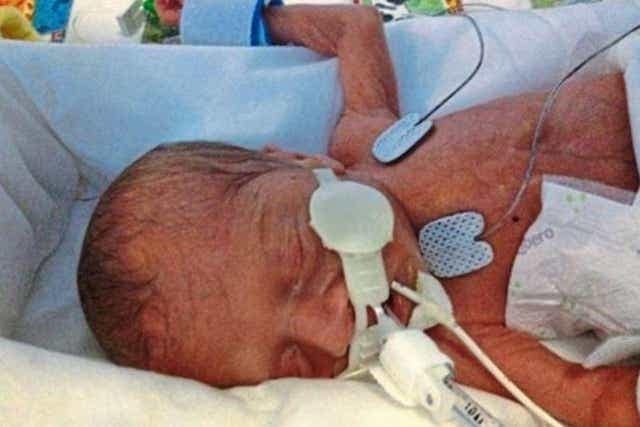
The babies, who were all undergoing care in hospital after being born premature, died after receiving total parenteral nutrition (TPN) feed contaminated with Bacillus cereus.
Three-month-old Aviva Otte, one-month-old Oscar Barker and nine-day-old Yousef Al-Kharboush were being provided critical nutrition via TPN, and an inquest at Southwark Coroner’s Court included the Bacillus cereus contaminant in each of their causes of death.
The exemption under the Medicines Act 1968 allows organisations to legally produce bespoke medicines without a licence for specific patients facing niche problems.
Yousef, who also died at St Thomas’ Hospital but in June 2014, and Oscar, who died at Addenbrooke’s Hospital in Cambridge in the same month, received TPN produced by a “commercial provider” – ITH Pharma – which is a licensed provider and not subject to the section 10 exemption.
In his PFD report – sent to the Department of Health, NHS England, the Care Quality Commission (CQC) and the Medicines, and Healthcare Products Regulatory Agency (MHRA) – Dr Morris highlighted concerns about what obligations section 10 exempt entities are under to report “adverse events”.
He said: “There is no requirement for a section 10 exempt entity to report any of its findings to the MHRA or indeed to other Trusts or the industry in general if an adverse event occurs.
“There may be times when section 10 entities reach conclusions which would assist the wider industry and help to assist both other Trusts and commercial organisations in assessing their own risks and improving the provision of highly specific medication to a group of vulnerable patients.
“The same may also be true of commercial organisations but they have the power of the MHRA controlling and effecting recalls and actions and the wider dissemination of information.”
The senior coroner also wrote that Bacillus cereus is resistant to some cleaning methods and that “sporocides” can be required to achieve decontamination.
“This was information and a conclusion that the Trust had reached in early 2014 and therefore prior to the outbreak in May/June 2014,” Dr Morris said.
He said there is a risk that future deaths could occur unless action is taken in respect of the highlighted concerns.
Recipients have to respond to the report by January 8 next year.
ITH Pharma was fined £1.2 million by a crown court in 2022 after providing TPN from which 19 premature babies became infected across nine hospitals in 2014.
The company pleaded guilty to a single regulatory offence of failing to have a suitable and sufficient risk assessment, under the Management of Health and Safety at Work Regulations 1999, and to two regulatory offences under the Medicines Act 1968 of supplying a medicinal product on May 27 2014 not of the nature or quality specified in the prescription
“Any information that had been shared with ITH and the MHRA as a result of a previous outbreak in the NHS five months prior to the ITH incident could have been of real value in taking steps to prevent future possible incidents.
“We are proud to work with and support the NHS and importantly, patients in this vital work of specialised feeding systems.
“We offer our deepest sympathies to all the families affected by the events of 10 years ago.
“We have done everything possible to assist the Senior Coroner in ensuring these inquests examined the full picture of both outbreaks in 2014 and we hope that these findings provide the families with answers.”