Scientists around the world are racing to develop and test a Covid-19 vaccine.
Whichever vaccine passes regulatory hurdles first is set to be the fastest ever vaccine developed.
But does this speed mean that safety will be compromised?
The Medicines and Healthcare Products Regulatory Agency (MHRA) said there is “absolutely no chance” that it will compromise on safety standards in order to rush out a vaccine.
Here are your questions answered about the history of vaccine development and safety.
– Why are scientists working at such speed?
The new coronavirus virus has spread to almost every corner of the planet – more than 51 million people have been infected which has led to more than 1.2 million deaths.
But the Covid-19 pandemic has not only had a devastating impact on human health, it has also severely impacted global economies.
“We’ve got a global pandemic and there’s a need to respond and so the vaccine community has really moved very, very quickly. And the reason it’s moving so quickly, is the health crisis, and also the economic crises caused by the pandemic.”
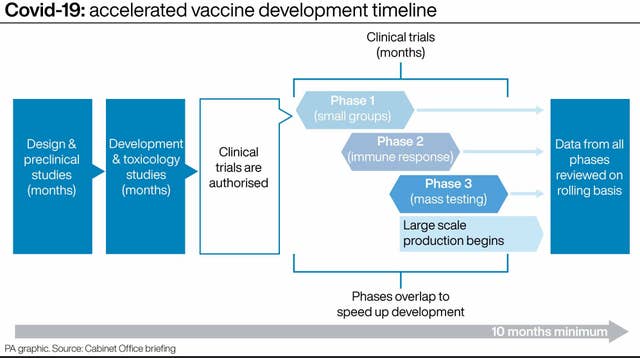
– How are vaccines being developed so quickly?
Prof Dougan added: “The actual disease itself, and the antigen which is used, or the piece of the virus that’s used for protection, is well definable. So that allows [scientists] to get off to a rapid start to set the normal processes in motion.”
He said there was an “unprecedented field of candidates”- there are nearly 200 vaccine candidates in trials.
Dr Claire Waddington, clinical lecturer in infectious disease at Cambridge University, told PA: “Some of it is reflecting modern science in that, certainly for the Oxford vaccine, they basically had a vaccine, almost ready to go where you could just plug in the genetic code. But the background to the vaccine was already established and being used for other things.
“And that’s in vast contrast to when vaccines were developed 40, 50 years ago where essentially we didn’t have any of that genomic knowledge or the sort of scientific capability to do such specific planning.
“With our ability to do things like genome sequencing, we’re able to quickly move to much more specific information that we can use to develop a vaccine. And it’s also allowed that kind of background technology to develop.”
– Will it be the quickest vaccine ever developed?
There have also been other illnesses – such as Ebola – which have seen rapid vaccine development.
But experts believe that this could be the speediest ever.
Dr Waddington added: “It’s usually in a realm of about 10 years to develop a vaccine. I think the record is around, maybe three or five years.”
– Will safety be compromised?
England’s deputy chief medical officer Jonathan Van-Tam said the standards are “no lower” because this is a public health emergency.
In a Downing Street data briefing, Professor Van-Tam set out how
the timetable for developing and approving a vaccine had been condensed from the usual number of years due to the coronavirus crisis.
Prof Van-Tam said: “Everyone knows that this is a public health emergency. We are in a much more difficult position.”
But he said that the three phases of clinical trials were no smaller and “the
standards are no lower just because this is a public health emergency”.
He described how data from each phase of the clinical trial can be put to regulators in what has been called a “rolling review” – instead of regulators going through reams of information at the conclusion of the trials.
– What does that mean for regulation?
The new way of working means that regulators around the world can start to look at scientific data earlier than they traditionally would do.
British regulators said the safety of the public always come first.
Dr June Raine, chief executive of the MHRA, told the Downing Street briefing: “A Covid-19 vaccine will only be approved once it has met robust standards of effectiveness, safety and quality.”
“Our teams of scientists and clinicians carefully, methodically, scientifically, rigorously review all the data on safety, effectiveness and quality.
“And we ask questions, what does the vaccine contain? How does it work in the body? What level of protection does it provide?
“The public can be confident that all those tests are done to the very highest standards. Safety is our watchword.”
Dr Raine added: “This is a critically important point.
“Although we have adapted our processes to undertake our rigorous review of effectiveness and safety in a rolling way, there is absolutely no chance that we will compromise on standards of safety or effectiveness.
“The steps that help to reassure on that include our independent expert advisory committee, which is an even greater layer of independence.”
– How do you monitor safety for new medicines?
Prof Dougan told PA: “What would normally happen is that as you develop a new medicine, or a new vaccine, is that when you administer the vaccine for the first time, you often get signals very early on – so you know yourself when you’ve had a vaccine sometimes because you get a little reaction to it.
“And so a lot of the events to do with safety are very early events and they’re sort of obvious.
“Then you have these things what we call adverse events that are actually rarer. So as you increase the number of people who you vaccinate you will then identify individuals in the population that will have an ‘unusual setup’ either because of the clinical condition or the genetic setup, and these are often what we call rare events but you’ll pick them up as you go through the process towards phase three (clinical trials).
“And then what you would normally do is you would also monitor a vaccine after licencing. And then look for the even rarer events or any longer term events that you might see.”






