A host of European countries have announced they will resume using the AstraZeneca vaccine after the continent’s regulator concluded it was “safe and effective”.
French Prime Minister Jean Castex said the country will begin vaccinating people again with the Oxford/AstraZeneca vaccine from Friday, while Italy expects to start on the same day.
Germany has also said it will return to administering the Oxford/AstraZeneca jab on Friday, following the European Medicines Agency (EMA) pronouncement.
Spain, Portugal and the Netherlands said they will follow suit next week, although Spain said it could exclude certain groups.
Emer Cooke, EMA executive director, said this situation was not unexpected, adding that “when you vaccinate millions of people” such reports of rare events will occur.
But the EMA has concluded there is no overall increase in the risk of blood clots with the vaccine, and in fact it is likely to reduce the overall risk of clots.
Ms Cooke, who said she would personally take the vaccine, told a press briefing: “The committee has come to a clear scientific conclusion.
“This is a safe and effective vaccine. Its benefits in protecting people from Covid-19, with the associated risks of death and hospitalisation, outweigh the possible risks.
“The committee also concluded that the vaccine is not associated with an increase in the overall risk of thromboembolic events, or blood clots.”
Peter Openshaw, professor of experimental medicine at Imperial College London, said the decision to pause use of the Oxford jab in some European countries was likely to “translate into many, many lives lost due to Covid”.
I welcome the growing number of European countries restarting the use of the Oxford AstraZeneca vaccine.
The vaccine is safe, effective & saving lives.
— Matt Hancock (@MattHancock) March 18, 2021
He told Times Radio that the delay and the “uncertainty” caused by the move will increase the number of coronavirus deaths by “thousands”.
Health Secretary Matt Hancock said he welcomed the “growing number of European countries restarting the use of the Oxford AstraZeneca vaccine”.
“The vaccine is safe, effective and saving lives,” he tweeted.
Ann Taylor, AstraZeneca’s chief medical officer, said she hoped vaccinations could resume “across Europe” following the conclusions of the EMA and the UK’s Medicines and Healthcare products Regulatory Agency (MHRA).
The MHRA concluded that any link between the jab and clots is unproven and the benefits of the AstraZeneca vaccine outweigh any risks.
Ms Taylor said: “Vaccine safety is paramount and we welcome the regulators’ decisions which affirm the overwhelming benefit of our vaccine in stopping the pandemic.
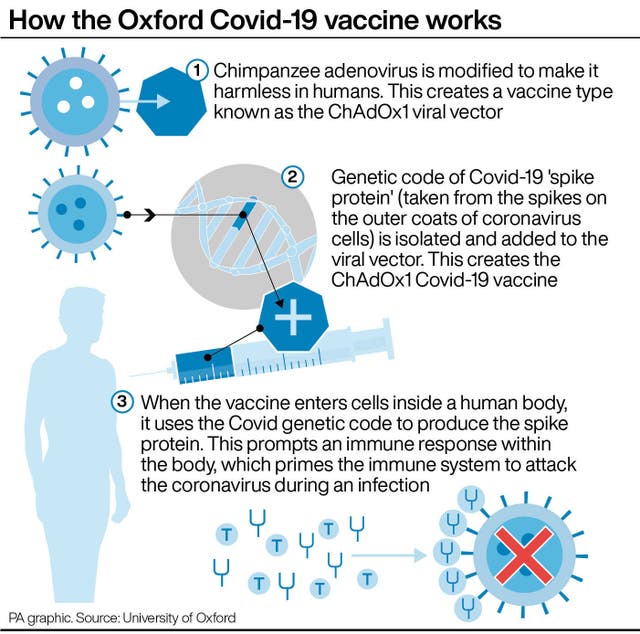
It comes after it emerged five men in the UK have suffered an “extremely rare” blood clot problem after having the AstraZeneca coronavirus vaccine, though no causal link with the jab has been established.
The men, aged 19 to 59, have experienced a specific type of blood clot in the brain together with low blood platelet count. One of the five has since died.
The MHRA said it was looking at the reports but stressed the events were “extremely rare” and there was a possibility they could have been caused by Covid itself.
It said the cases represented a less than one-in-a-million chance of suffering this type of clot among those who have been vaccinated, while the risk of dying from Covid aged 40 to 49 is one in 1,000.
The type of clot – cerebral venous sinus thrombosis (CVST) – prevents blood from draining out of the brain.
Dr June Raine, MHRA chief executive, told a Downing Street press conference that, as a precautionary measure, the MHRA advised “anyone with a headache that lasts more than four days after vaccination or bruising beyond the site of vaccination after a few days to seek medical attention”.
He said there were “anecdotal reports” of small numbers of people not turning up for vaccine appointments following the controversy, but he expected many of those would decide to get the jab after “a pause for thought”.
It is the CVST clot that led Germany to halt its rollout of the AstraZeneca vaccine, prompting other countries across Europe, including Italy and France, to follow.
Officials in Germany received six reports of CVST associated with low blood platelets – all in younger to middle-age women.
The EMA said the committee had recommended “raising awareness” of possible risks associated with the AstraZeneca vaccine and ensuring they are included in the product information for the vaccine.
Around 20 million people in the UK and Europe had received the vaccine as of March 16 and the EMA had received reports of just seven cases of blood clots in multiple blood vessels and 18 cases of CVST, it said.