Public Health England (PHE) has defended the use of the AstraZeneca vaccine after German advisers said there was insufficient data to recommend it for those aged 65 and over.
Dr Mary Ramsay, head of immunisations at PHE, said there had been “too few cases” of coronavirus in older people in Phase 3 clinical trial to determine efficacy in this age group, but other data on immune response had been “reassuring”.
It comes after a draft recommendation from Germany’s vaccination advisory committee said the jab should only be given to people aged 18 to 64 for now.
It added: “There currently is not sufficient data to assess the vaccination effectiveness from 65 years.”
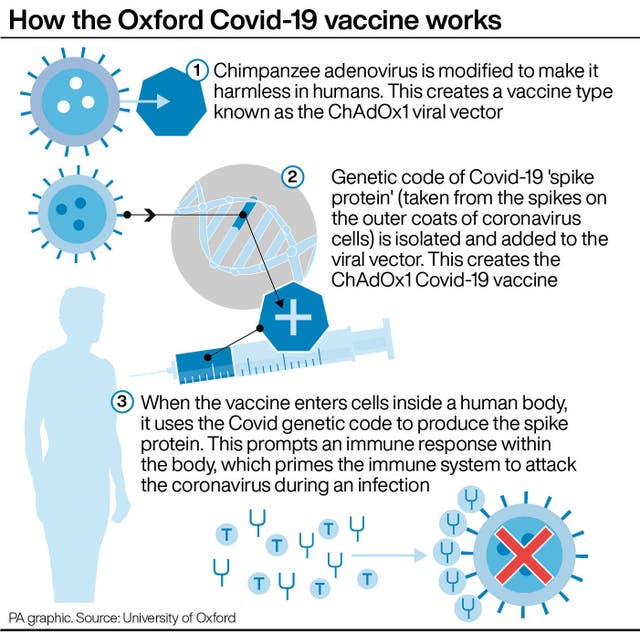
Dr Ramsay said: “Both the AstraZeneca and Pfizer/BioNTech vaccines are safe and provide high levels of protection against Covid-19, particularly against severe disease.
“There were too few cases in older people in the AstraZeneca trials to observe precise levels of protection in this group, but data on immune responses were very reassuring.
“The risk of severe disease and death increase exponentially with age – the priority is to vaccinate as many vulnerable people as possible with either vaccine, to protect more people and save more lives.”
Oxford University, which partnered with AstraZeneca to develop the vaccine, has stressed that its jab offers high protection against severe disease and prevents people needing to go to hospital.
In that particular analysis, only 12% of people given two doses of the vaccine in the UK arm of the trial (285 out of 2,377) were aged 56 to 69, while 9% (213) were over 70.
Some 12% of people in the control group (given a dummy vaccine) were also aged 56 to 69 while 9% were over 70.
Older people made up similar proportions in the Brazilian section of the trial, which was made up of 4,088 people.
Previous work published in November included findings for 560 people. Of these, 160 were aged 18 to 55, 160 were aged 56 to 69, and 240 were 70 or older.
Those results found that all age groups, including older people, had an immune response to the vaccine after two doses.

AstraZeneca and the vaccines minister Nadhim Zahawi dismissed the report as “incorrect” and “untrue”.
Meanwhile, Downing Street said the UK’s chief scientific adviser, Sir Patrick Vallance, had told the Cabinet that the Oxford/AstraZeneca vaccine had been shown to be effective in younger and older people.
A No 10 spokesman said: “The chief scientific adviser set out that the Oxford/AstraZeneca vaccine remains both safe and effective and that the trials showed similar immune responses in both younger and older adults.”






